The Birth of Astrophysics
Liv Büsser (5C) 02 Feb 2021
The discovery and development of spectral analysis brought a completely new quality to astronomical research. Since its application, scientists no longer only observe celestial objects, but also analyse them for their chemical composition. Spectral analysis laid the foundation for the science known today as astrophysics. There is hardly any difference between astronomy and astrophysics in the scientific world today.
Many years ago, when technology was so advanced that it was possible to study the composition of celestial bodies, a big difference was made between astronomy and astrophysics. Astronomy was concerned with the investigation of celestial bodies and the universe. Astronomy is one of the oldest sciences and is strongly influenced by the fields of mathematics, physics and computer science. Astrophysics, on the other hand, is a branch of physics and deals with the exploration of space using physical methods (e.g. spectral analysis). It is more concerned with the composition of planets, stars or galaxies. In a survey, organised by Florian Freistetter in 2011, astronomers/astrophysicists and people without astronomical/astrophysical background took part. The survey was based on the following three questions: Do people today still see a difference between astronomy and astrophysics? Which subject is more difficult, astronomy or astrophysics? Would one rather recommend an astronomer or an astrophysicist to a company/organisation? On the first point the majority sees a difference between astronomy and astrophysics. However, it is mainly people without an astronomical background who make this claim. In fact, almost all the astronomers and astrophysicists surveyed see no difference between the two subjects.
A second survey was about which subject is more difficult. Astronomy or astrophysics? The result was that almost everyone rated both subjects as equally difficult.
In the last point the question was asked whether a certain company/organisation would rather employ an astronomer or an astrophysicist or whether it didn't matter.
The results show that:
- 30% would rather take an astrophysicist as a teacher than an astronomer (8%),
- 39% would be more likely to use astrophysicists rather than astronomers (3%) in the financial sector and
- 47% find astrophysicists at NASA more useful than astronomers (5%).
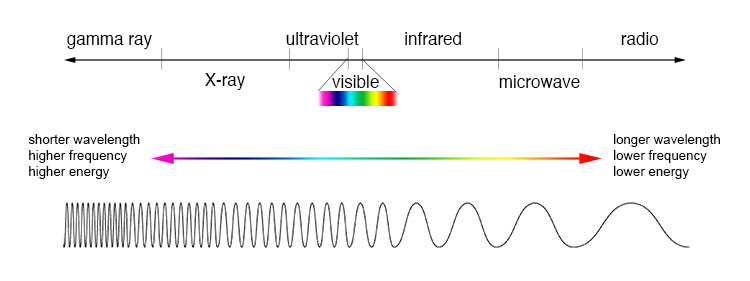
Let us now come back to what caused the birth of astrophysics: Spectral analysis. To understand the principle of spectral analysis you need to know what a spectrum is. The electromagnetic spectrum covers the entire range of wavelengths of all known electromagnetic radiation from gamma rays to visible light, infrared, radio waves and the low-frequency range. An electromagnetic wave consists of electric and magnetic fields that are perpendicular to each other and to the direction of propagation. The oscillation process consists of a periodic change in the electric and magnetic field strength. Both oscillate in the same rhythm.
With the help of spectral analysis, astronomers collect information about the chemical composition of various celestial bodies. It was developed in the 19th century by Robert Wilhelm Bunsen and Gustav Kirchhof. Spectral analysis is based on the discovery of Joseph von Fraunhofer. Using a glass prism, he found out that the spectrum of sunlight had dark lines in various places. If white light is directed through a glass prism, the light rays are refracted to different degrees depending on their wavelength.
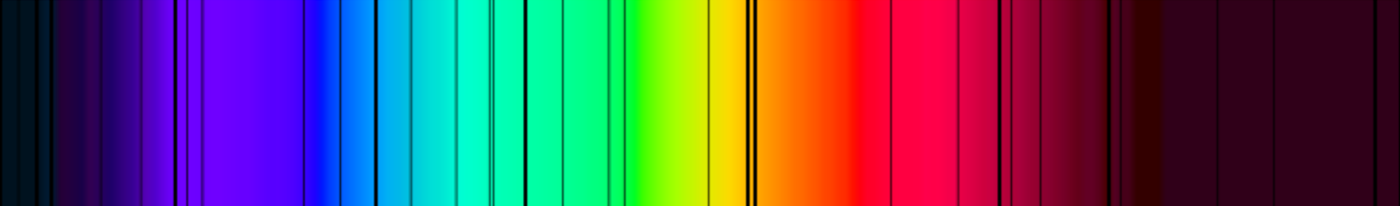
Electromagnetic waves are created by electrical charges that move with irregular motion. They are disturbances that propagate through space. Each wavelength corresponds to a specific colour. This is why the colour spectrum becomes visible in fanned out light. Fraunhofer noticed that there are dark lines in the spectrum of sunlight and in the spectrum of different stars. These lines are called absorption lines. They come from the fact that certain elements in the stellar envelope absorbs light with a very specific wavelength. Each chemical element has an individual, very characteristic fingerprint. Scientists use the spectral lines to determine which elements are present in a star.
On the other hand, there are also the so-called emission lines in the spectrum. They are created when chemical elements are stimulate and emit light of a certain wavelength, which is then reflected in the spectrum in the form of particularly bright lines. If absorption and emission lines in a spectrum are precisely analysed, it is possible to make precise statements about the presence of chemical elements in astronomical objects - even over long distances.
Links
- https://de.wikipedia.org/wiki/Spektroskopie#Spektroskopie_in_der_Astronomie
- https://de.wikipedia.org/wiki/Sonnenstrahlung#Sonnenspektrum
- https://de.wikipedia.org/wiki/Spektralklasse
- https://www.spektrum.de/lexikon/physik/absorptionslinie/137
- https://www.studieren-studium.com/studium/fachbereich_vergleichen/Astronomie/Astrophysik
- https://scienceblogs.de/astrodicticum-simplex/2011/11/25/astronomie-vs-astrophysik/
Cover image: Computer rendering of the Chandra X-Ray Observatory (Credits: NASA).